Updated 24 Nov 2021.
The up-to-date version of this protocol is here.
¶ Protocol Snapshot 23 Nov 2021
¶ Materials
For BosLab Osmotic Stress Minimal Medium version 0.1 (BLOSMM v0.1) as made on 23 Nov 2021 BosLab Novice Night
| Desired composition | for 500 mL final volume of BLOSMM v0.1 |
|---|---|
| 0.01% Miracle-Gro Water Soluble All Purpose Plant Food (Miracle-Gro product 1001123) | 0.05 g Miracle-Gro powder: which is difficult to measure accurately, so I recommend making 10X stock solution by adding enough distilled H2O to 1 g Miracle-Gro to make 1 L, then aliquoting 50 mL for this recipe |
| 1% glucose | 5 g glucose (in BosLab the bottle is labeled “dextrose”) |
|
0.6 M NaCl intended but 1.2 M NaCl actual. Drat! Math error. I put 35.1 g NaCl into a 500 mL volume so I mistakenly made a 1.2 M NaCl concentration, twice the intended 0.6 M! |
17.55 g NaCl intended but 35.1 g NaCl actual |
| 0.1X LB (a.k.a. lysogeny broth, Luria-Bertani broth) | 50 mL LB |
| distilled H2O | enough to make 500 mL final volume |
For 1 mole/liter trehalose dessication solution (1 M trehalose)
| Desired composition | for 500 mL final volume of 1 M trehalose |
|---|---|
| 1 M trehalose | 189.1 g trehalose dihydrate |
| distilled H2O | enough to make 500 mL final volume |
- Liquid LB media, at least 1 mL per E. coli strain we're drying
- Two 1 L culture media bottles (make/autoclave BLOSMM v0.1 and 1 M trehalose)
- DH5α E. coli stock
- Agar art E. coli stocks
- 14 mL culture tubes, two per E. coli strain we're drying
- Dry culture carrier comprising either:
- sterile petri dishes and pieces of filter paper cut to fit in them, or
- new unused zip lock bags and pieces of filter paper cut to fit in them
- 37°C plate incubator to use for drying cells (consider drying at 30°C if cells don't revive)
- 37°C shaker incubator for liquid cultures
¶ Procedure
TBD, but try something like:
Make and autoclave BLOSMM v0.1.
Make and autoclave 1 M trehalose.
Grow each strain in 3 mL BLOSMM v0.1 added to 3 mL autoclaved deionized H2O in a sterile culture tube and measure OD to determine when cells are in log‑phase growth.
- Ask Wendy how we measure OD at BosLab.
- Alas, our OD reading machine uses non-sterile cuvettes with a minimum volume requirement of 0.6 mL. Hence we will need to throw away 0.6 mL of cell culture every time we take a reading.
- This is why I chose a total culture volume of 6 mL: it allows us to make 5 readings yet still have half the culture remaining.
- Does E. coli successfully grow in 1:2 diluted BLOSMM v0.1?
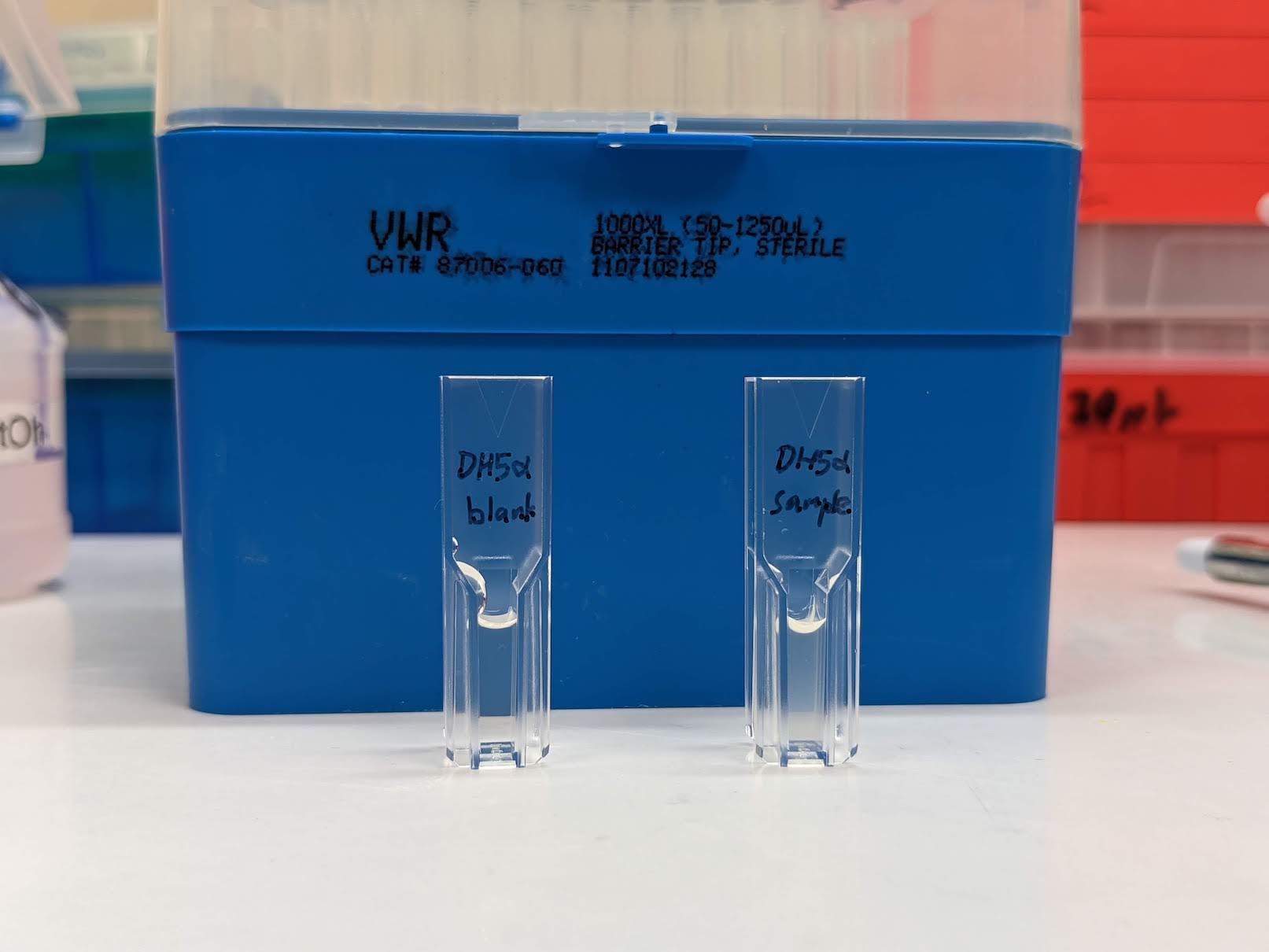
- On 23 Nov 2021 we got this far into this protocol, but consistently measured OD600 = 0.00 Abs for both the DH5α and Agar Art GPP strains over 2.5 hours of incubation.
- So looks like the cells aren't growing in BLOSMM v0.1.
We never got to the following steps.
Centrifuge to pellet cells. Discard supernatant.
Resuspend cells in 1 mL of 1 M trehalose.
Pipette 100 µL per storage spot on the filter paper. (Should the filter paper have storage spots marked then autoclaved ahead of time?)
Put paper in sterile petri dishes with lids closed but NOT parafilmed or sealed. Or put paper in new unused zip lock bags (leave bags open).
Place petri dishes or bags in plate incubator to dry. May take 1-3 days depending on ambient humidity.
Remove petri dishes and/or bags and store in a cool dry place.
TBD: rehydration procedure using LB.
¶ Results
24 Nov 2021 Wednesday: Came in and found that the DH5α culture tube had grown cloudy but the GFP tube stayed clear, implying that the DH5α grew but the GFP strain didn't. Measured OD600 = 0.37 Abs for the DH5α but didn't measure the GFP tube because I didn't feel like wasting cuvettes on visually clear culture media. Speculations as to what to try next? Maybe directly inoculating minimal media like BLOSMM with −20°C glycerol cultures isn't such a great idea. Considering starting the cultures next time in LB until visible growth, then subculturing in BLOSMM.